Abstract
Introduction
Primary lymphomas of the central nervous system (PCNSL) have recently benefited from therapeutic progress but after the 2nd line the prognosis is particularly poor, with a survival of less than 6 months. CAR-T cells are of major contribution for systemic lymphomas, but the indication in PCNSL is prohibited in the United States, in Europe the prohibition is not specified. Since 2020, French centers, within the French national LOC network, have treated PCNSL in 3rd line and above. We present here the real-life results of the use of commercial CAR-T cells in PCNSL and compare them to the results of patients in the LOC network who did not benefit from them.
Methods
We retrospectively selected from the French LOC network database the adult immunocompetent PCNSL patients treated with CAR-T cells from the 3rd line of treatment. As control, we studied PCNSL patients from the LOC database treated with any treatment, at least in 3rd line and considered not eligible for an autologous stem cell transplantation (ASCT) or in relapse after ASCT.
Results
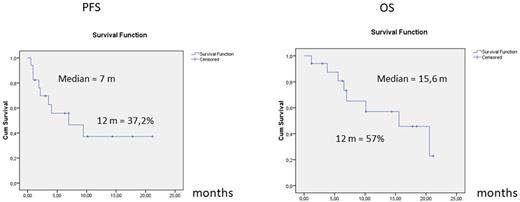
17 PCNSL patients (median age : 68, median Karnofsky performans status (KPS) : 80, 9 women, 8 men) were treated with CAR-T cells (tisa-cel : N=13, axi-cel : N=4) between May 2020 and May 2022. They had previously received a median of 3 lines of treatment, including ASCT in 10/17 cases. 13 had a cerebral involvement (+/- eye and CSF) and 4 an isolated CSF involvement. All but one patient received a bridging therapy before CAR-T cells, resulting in complete response (CR) (N=1), partial response (PR) (N=6), stable disease (SD) (N=3), progressive disease (PD) (N=7). The median follow-up after CAR-T cells was 17.8 months. Best response was CR in 9 patients, PR in 4 patients and PD in 4 patients (overall response rate (ORR) of 76%). Median progression-free survival (PFS) was 7 months and median overall survival (OS) was 15.6 months. 15/17 patients experienced a CRS, including one grade III-IV, 9/17 an ICANS, including 3 grade III-IV, and 5/15 grade III-IV cytopenia lasting more than 28 days. In the control group (N=247), median age was 68 and median KPS 60. The efficacy endpoints were significantly worse in the control group compared to the CAR-T group: ORR of 41% (p=0.004), median PFS of 2.9 months (p=0.04) and median OS of 4.8 months (p=0.02).
Conclusion
CAR-T cells are effective in PCNSL, with responses and toxicity close to those found in systemic NHL. In comparison with LOC network data, this processing significantly improves PFS and OS. Data on a larger number of patients will make it possible to define prognostic factors for response and refine the indications.
Disclosures
Sylvain:Novartis: Consultancy, Honoraria; gilead: Consultancy, Honoraria. Le Garff-Tavernier:Abbvie: Consultancy, Honoraria; Janssen: Honoraria. Willems:Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees. Houot:Kite/Gilead, Novartis, Incyte, Janssen, MSD, Takeda, Roche: Honoraria; Kite/Gilead, Novartis, BMS/Gelgene, ADC THerapeutics, Incyte, Miltenyi: Membership on an entity's Board of Directors or advisory committees. Soussain:Gossamer Bio: Other: travel funding ; AstraZeneca: Research Funding; Hangzhou Henzen Pharmaceuticals: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal